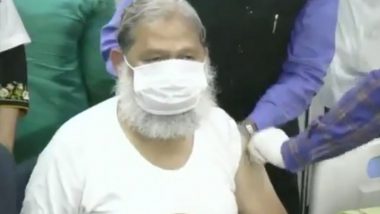
New Delhi, December 5: Days after receiving a trial dose of Covaxin, a potential vaccine against coronavirus (COVID-19) developed by Bharat Biotech, Haryana Health Minister Anil Vij tested positive for the virus. Anil Vij, who has been admitted to a hospital, urged all those who had come in contact with him recently to get tested. He was administered a trail dose of Covaxin on November 20. Bharat Biotech Says 'Anil Vij Didn't Receive Second Dose of COVID-19 Vaccine Covaxin, Efficacy Determined 14 Days Post 2nd Shot'.
Vij recieved a trial dose of Covaxin as part of the vaccine's third phase of trials. After Vij tested positive for coronavirus days after receiving Covaxin, it is natural that doubts will be raised over the vaccine's efficacy and safety. But, one must remember that Vij was given Coviaxin as part of the ongoing trials of the vaccine. And trails are conducted to ascertain its efficacy as well as its adverse impacts. Pfizer COVID-19 Vaccine: Why is Getting The Vaccine a Challenge For India? AIIMS Delhi Director Randeep Guleria Explains.
Another thing that many are missing is that the second dose of Covaxin was pending and Vij hasn't recieved it. Covaxin has a two-dose schedule at a 28-day interval. He only received the first dose. Therefore, it can not be concluded that the vaccine failed. Moreover, such incidents don't necessitate stoppage of vaccine trials or its rollout. Again, trials are conducted so that a vaccine can be evaluated on all parameters.
There is a possibility that Vij was given a placebo and not the actual dose of the vaccine. For example, 10 severe cases of COVID-19 were observed during the trial of Pfizer's vaccine BNT162b2. Of the 10 cases, 9 occurred in the placebo group and one in the BNT162b2 vaccinated group. "The phase-3 trials are double-blinded and randomized, where 50 percent of subjects (participants in the trial) receive vaccine and 50 percent of subjects receive placebo," Bharat Biotech has said.
Efficacy of a vaccine won't become a hurdle in its rollout given the grave situation the world is facing due to the pandemic. Remember, vaccines against Malaria are only 30 percent protective and yet there were launched. All COVID-19 vaccines are all above 90 percent in their effectiveness. There's a lesson for the government and vaccine-makers as well.
When a volunteer develops some health-related issues after receiving a trial dose, the incident leads to panic and can discourage people from getting vaccinated. Therefore, the participation of volunteers should be confidential. But, their safety must be paramount and they must be compensated adequately if they develop any illness.
(The above story first appeared on LatestLY on Dec 05, 2020 01:42 PM IST. For more news and updates on politics, world, sports, entertainment and lifestyle, log on to our website latestly.com).












 Quickly
Quickly